1 - PRINCIPLES OF CHEMISTRY
 States of matter -
States of matter - Solids -
- strong forces of attraction between particles - close together
- fixed positions
- lattice arrangement
- particles don't move - solids keep definite shape and volume
- particles vibrate about their positions
- hotter = more vibration = slight expansion

Liquids -
- weak force of attraction between particles
- randomly arranged
- free to move past each other
- generally stay closely together
- definite volume but not definite shape
- flow to fill bottom of a container
- constantly moving with random motion
- hotter = move faster = slight expansion
- very weak force of attraction between particles
- free to move
- far apart
- particles travel in straight lines (bounce off objects/each other)
- no definite shape or volume
- fill any container
- move constantly with random motion
- hotter = move faster = expand/increase pressure
Changes of state -
HEAT ENERGY SUPPLIED -
Solid - Liquid -
1. Solid heated, particles gain energy
2. Particles vibrate more, weakens bonds
3. Expands
4. Particles gain enough energy to break free from positions
MELTING
5. Liquid
Liquid - Gas -
1. Liquid heated, particles gain more energy
2. Particles move faster, weakens and breaks bonds holding it together
3. Particles gain enough energy to break bonds
EVAPORATING
4. Gas
HEAT ENERGY GIVEN OUT -
Opposite happens!
Gas - liquid -
CONDENSING
Liquid - solid -
FREEZING
Movement of particles -
Diffusion -
- The gradual movement of particles from areas of higher concentration to areas of lower concentration
 Diffusion experiment -
Diffusion experiment -1 - Potassium manganate(VII) and water
- Beaker of water with potassium manganate(VII) at the bottom
- Purple colour diffuses into the rest of the beaker
- Random motion of particles in a liquid - causes purple colour to spread out
- Adding more water would dilute the potassium manganate(VII) = less purple
- Aqueous ammonia (NH3) gives off ammonia gas
- Hydrochloric acid (HCl) gives off hydrogen chloride gas
- Set up a glass tube with a bung in each end - cotton wool soaked in aqueous ammonia at one end and cotton wool soaked in hydrochloric acid in the other end
- Gases diffuse4 from each end of the tube
- Ammonia gas diffuses faster (smaller and lighter particles) - white ring of ammonium chloride forms closer to the hydrochloric acid

3 - Bromine gas and air
- Bromine gas is brown and strongly smelling
- Fill half a gas jar with bromine gas
- Fill other half with air (keep gases separate with glass plate)
- Remove glass plate
- Brown bromine gas diffuses through the air
Atoms -
1 - Nucleus
- Middle of atom
- Protons and neutrons
- Positive charge
- Contains most of the mass of the atom
- Move around nucleus in shells
- Negatively charged
- Cover a lot of space
- Size of orbit depends on size of atom
- Virtually no mass
 Protons = heavy and positive
Protons = heavy and positiveNeutrons = heavy and neutral
Electrons = small mass, negative
Number of electrons = number of protons (in neutral atom/not isotope) -
- Neutral atoms = no charge overall
- Charge on electrons is same size as the charge on protons (but opposite)
- Electrons added/removes - atom becomes a charged ion
- Atomic number = no. of protons
- Mass number = protons + neutrons
- No. of neutrons = mass number - atomic number
- Groups of atoms (joined together)
- Can be one element (H2, N2) or more than one element (H2O, CO2)
- Held together by covalent bonds
1 - Elements
- Made up of one type of atom only
- Chemically bonded
- 2 or more different elements - bonded together
- eg: carbon dioxide - 1 carbon atom bonded to 2 oxygen atoms = molecule of carbon dioxide
- Difficult to separate original elements again
- Properties of the compound generally very different from properties of the original elements
- eg: iron sulphide
- Easily separates
- No chemical bond between different parts of the mixture
- Can be separated by physical methods (eg: distillation)
- eg: air - mixture of gases (nitrogen, oxygen, carbon dioxide, argon), can all be separated out easily
- Crude oil - mixture of different length hydrocarbon molecules
Crystallisation -
1. Pour solution into evaporating dish
2.Heat solution - some solvent evaporates, solution gets more concentrated
3. Stop heating when crystals start to form
4. Remove dish from heat, put in warm place for rest of solvent to evaporate
5. Dry product - drying oven or desiccator
Separating rock salt -
- Mixture of salt and sand
- Salt and sand are compounds
- Salt = soluble - dissolves then forms as crystals
- Sand = insoluble - collects on filter paper
2. Dissolve in beaker and stir
3. Filter through filter paper in funnel - filtering
4. Evaporate in evaporating dish - crystallisation
Distillation -
1 - Simple distillation
- Separating out a liquid from a solution
- Not similar boiling points
- Can use to separate pure water from seawater - evaporate until just salt left in flask

2. Lowest boiling point evaporates
3. Vapour cooled, condenses and is collected
4. Rest of solution left in flask
2 - Fractional distillation
- Mixture of liquids
- Similar boiling points
- Used for fractional distillation of crude oil at a refinery

2. Fractionating column on top
3. Heat
4. Liquids evaporate at different temperatures
5. Lowest boiling point - when thermometer reaches its boiling point it will be a the top of the column
6. Condensing through tube and collects into test tubes
Chromatography -
- Separates out dyes
- Can help you identify dyes - compare to what you think it might be
- Different dyes move up the paper at different rates

2. Add spots of different dyes on the line at regular intervals
3. Put sheet in beaker of solvent, not touching the line (eg: water - depends on what is being tested, sometimes ethanol is needed)
4. Put lid on container - stop solvent evaporating
5. Solvent carries dye up the paper and separates colours within it
Ionic bonding -
- Atoms lose or gain electrons to form charged particles (ions)
- Strongly attracted to one another because of the opposite charges - electrostatic attrcation
- High melting and boiling points
- Dot and cross diagrams (with square brackets and charge)
Gaining electrons = reduction
Shell less than half full of electrons -
- Left hand side of periodic table
- Want to become more stable like the noble gases
- Try to lose the electron - easier than completing shell
- Become positive
- Gives electron to oppositely charges atom wanting to complete shell - stick together
- Right hand side of periodic table (group 6 and 7)
- Gain extra electrons to complete shell
- Become negative
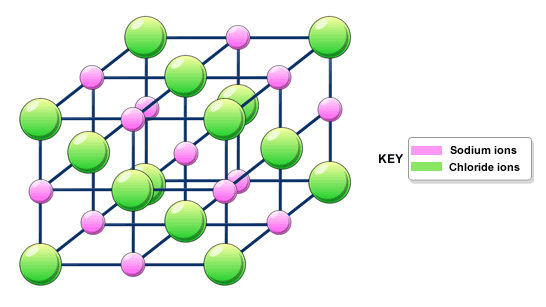
Giant ionic structures -
- Compounds with ionic bonding always have giant ionic structures
- Ions held together in closely packed 3D lattice (attraction between oppositely charged ions - very strpmg)
- A lot of energy is needed to overcome the strong forces of attraction - high melting and boiling points
- The more charged it is, the stronger the forces of attractions are
- Shared pair of electrons
- Both atoms have full outer shells
- Each atom has to have enough covalent bonds to fill up outer shell
- Strong attraction between the shared electrons and the nuclei of the atoms
 eg:
eg:Hydrogen and chlorine = hydrogen chloride (HCl)
Ammonia (NH3)
Nitrogen (N2)
Water (H2O)
Methane (CH4)
Ethane (C2H6)
Ethene (C2H4)
Simple molecular substances -
- Atoms within molecule held together by very strong covalent bonds
- Forces of attraction between molecules are very weak - intermolecular forces
- Melting and boiling points are very low
- Most are gases or liquids are room temperature
- 'Mushy' physical state - liquid/gas/easily melted solid
- No charged ions
- Atoms bonded to eachother by strong covalent bonds
- Lots of energy to break the large amount of bonds
- Very high melting and boiling points
- Don't conduct electricity - no delocalised electrons
- eg: diamond and graphite (carbon atoms)
 Diamond -
Diamond - - Each carbon atom forms 4 covalent bonds
- Very rigid giant covalent structure
- Hardest natural substance
Graphite -
- Each carbon atom forms 3 covalent bonds
- Creates layers
- Slide over each other
- Good lubricant
- Free electrons - only non-metal that is a good conductor








No comments:
Post a Comment